The pH of a buffer is determined by two factors; 1) The equilibrium constant Ka of the weak acid and 2) the ratio of weak base [A–] to weak acid [HA] in solution. 1) Different weak acids have different equilibrium constants (Ka). Ka tells us what proportion of HA will be dissociated into H+ and A– in solution.
Hence, What are three biological buffers?
The body’s chemical buffer system consists of three individual buffers: the carbonate/carbonic acid buffer, the phosphate buffer and the buffering of plasma proteins.
Consequently, How do buffers work? How do buffers work? Buffers work by neutralizing any added acid (H+ ions) or base (OH- ions) to maintain the moderate pH, making them a weaker acid or base.
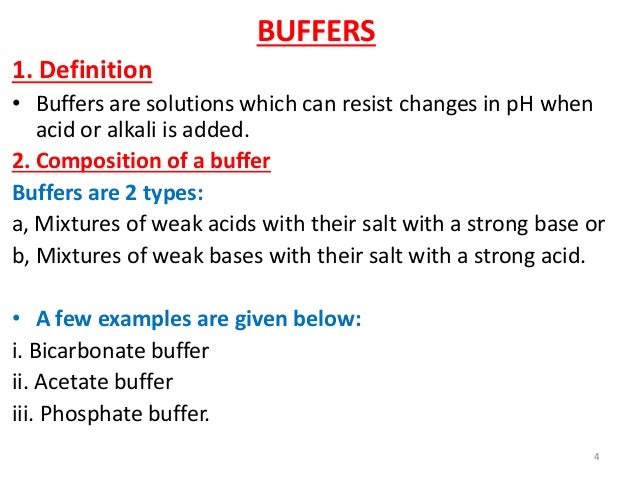
What are buffers made of? Buffers can be made from weak acids or base and their salts. For example, if 12.21 grams of solid sodium benzoate are dissolved in 1.00 L 0.100 M benzoic acid (C6H5COOH, pKa = 4.19) solution, a buffer with a pH of 4.19 will result: Buffers can be made from two salts that provide a conjugate acid-base pair.
In addition, What is pH full form? The full form of pH is Potential of Hydrogen. pH is known as the negative logarithm of H+ ion concentration. Hence the meaning of the name pH is explained as the strength of hydrogen. pH describes the concentration of the hydrogen ions in a solution and it is the indicator of acidity or basicity of the solution.
What is a buffer in the body?
A variety of buffering systems exist in the body that helps maintain the pH of the blood and other fluids within a narrow range—between pH 7.35 and 7.45. A buffer is a substance that prevents a radical change in fluid pH by absorbing excess hydrogen or hydroxyl ions.
Is an example of biological buffer?
A very commonly used biological buffer is called HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid). This buffer is very good at maintaining a steady pH between 6.8 and 8.2.
What are some buffers in the human body?
The three major buffer systems of our body are carbonic acid bicarbonate buffer system, phosphate buffer system and protein buffer system.
- Carbonic acid bicarbonate buffer system.
- Phosphate buffer system.
- Protein buffer system.
Why is a buffer important?
A buffer is a chemical substance that helps maintain a relatively constant pH in a solution, even in the face of addition of acids or bases. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis.
What is buffer and its types?
Buffers are broadly divided into two types – acidic and alkaline buffer solutions. Acidic buffers are solutions that have a pH below 7 and contain a weak acid and one of its salts. For example, a mixture of acetic acid and sodium acetate acts as a buffer solution with a pH of about 4.75.
How does a buffer maintain pH?
Maintaining the proper pH is critical for the chemical reactions; hence buffers are used. Buffer is the combination of a weak acid and the weak base. Hence when dissociated, H+ and OH- ions are released, which helps to stop the massive changes in pH levels and aids to maintain the balance.
How buffer is formed?
A buffer is made by mixing a large volume of a weak acid or weak base together with its conjugate. A weak acid and its conjugate base can remain in solution without neutralizing each other. The same is true for a weak base and its conjugate acid.
What is the pH of urine?
Normal Results
The normal values range from pH 4.6 to 8.0. The examples above are common measurements for results of these tests. Normal value ranges may vary slightly among different laboratories. Some labs use different measurements or test different samples.
What is the pH of blood?
The pH scale ranges from 0 (very acidic) to 14 (very alkaline). Blood is usually between 7.35 to 7.45.
What is human blood pH?
In the absence of pathological states, the pH of the human body ranges between 7.35 to 7.45, with the average at 7.40.
What is protein buffer?
Protein Buffers
Protein buffer systems depend upon proteins, as opposed to nonprotein molecules, to act as buffers and consume small amounts of acid or base. The protein hemoglobin makes an excellent buffer. It can bind to small amounts of acid in the blood, helping to remove that acid before it changes the blood’s pH.
Why is blood a buffer?
Human blood contains a buffer of carbonic acid (H2CO3) and bicarbonate anion (HCO3–) in order to maintain blood pH between 7.35 and 7.45, as a value higher than 7.8 or lower than 6.8 can lead to death. In this buffer, hydronium and bicarbonate anion are in equilibrium with carbonic acid.
What is the main buffer in our blood?
The Carbonic Acid-Bicarbonate buffer system is the most important buffer for maintaining the pH homeostasis of blood. In this system, gaseous metabolic waste carbon dioxide reacts with water to form carbonic acid, which quickly dissociates into a hydrogen ion and bicarbonate (see below).
What is buffer botany?
A chemical solution that counteracts small changes in pH when acids or alkalis are added to it. Buffers play an important role in cells and tissues, which usually function best at or near neutrality (pH 7) since changes in pH adversely affect metabolic processes.
Is hemoglobin a buffer?
The most significant buffer of blood is hemoglobin. Thus, Harper (1967), Guyton (1968), Slonim A. Hamilton (1976) and other authors believe that it accounts for 50-60 percent of the total buffer capacity of blood.
What substance acts as a buffer in organisms?
Examples of natural buffers are: carbonate and bicarbonate, phosphate, amino acids, and proteins.
What are the buffers in the human body?
The three major buffer systems of our body are carbonic acid bicarbonate buffer system, phosphate buffer system and protein buffer system.
What is buffer solution and pH?
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it.
What are the properties of buffer?
Characteristics of buffer solution
(i) It has a definite pH. (ii) Its pH does not change on standing for long periods of time. (iii) Its pH does not change on dilution. (iv) Its pH is slightly changed by the addition of small quantity of an acid or base.
What are physiological buffers?
A buffer is a solution that resists changes in pH. Since the efficiency of many enzymes and metabolic reactions is sensitive to pH, buffers are physiologically very important. A physiological buffer system usually consists of a weak acid and its conjugate base.