The purpose of a buffer in a biological system is to maintain intracellular and extracellular pH within a very narrow range and resist changes in pH in the presence of internal and external influences.
Hence, What is a buffer simple definition?
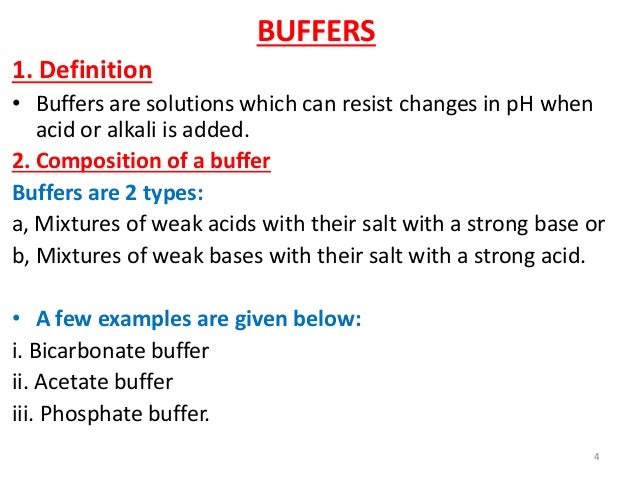
A buffer is a solution that can resist pH change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus maintaining the pH of the solution relatively stable.
Consequently, What is a buffer example? For example, a buffer can be composed of dissolved acetic acid (HC 2H 3O 2, a weak acid) and sodium acetate (NaC 2H 3O 2, a salt derived from that acid). Another example of a buffer is a solution containing ammonia (NH 3, a weak base) and ammonium chloride (NH 4Cl, a salt derived from that base).
What is a buffer in molecular biology? Buffers are aqueous solutions of weak acids and their conjugate bases, or weak bases and their conjugate acids. Buffers show little change in pH when small amounts of strong acids or bases are added. Buffer solutions are used in biological systems to keep pH at a nearly constant value.
In addition, What is a buffer in science? buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration.
What is a buffer and how does it work?
A buffer is simply a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers work by reacting with any added acid or base to control the pH.
What is a buffer in biology quizlet?
Buffer. a chemical solution that keeps pH within normal limits by absorbing excess hydrogen, or H+, ions and hydroxide, or OH-, ions.
What is the buffer system in the human body?
Human blood contains a buffer of carbonic acid (H2CO3) and bicarbonate anion (HCO3–) in order to maintain blood pH between 7.35 and 7.45, as a value higher than 7.8 or lower than 6.8 can lead to death. In this buffer, hydronium and bicarbonate anion are in equilibrium with carbonic acid.
What is a buffer made of?
A buffer is made by mixing a large volume of a weak acid or weak base together with its conjugate. A weak acid and its conjugate base can remain in solution without neutralizing each other. The same is true for a weak base and its conjugate acid.
What is the pH range of a buffer?
Buffers are generally good over the range pH = pKa ± 1. The ammonia buffer would be effective between pH = 8.24 – 10.24. The acetate buffer would be effective of the pH range from about 3.74 to 5.74. Outside of these ranges, the solution can no longer resist changes in pH by added strong acids or bases.
What is a buffer quizlet?
What is the definition of a buffer? A solution of a weak acid (proton donor) and its conjugated base (proton acceptor) that resists significant changes in pH upon addtion of small quantites of strong acid or base.
What’s the purpose of a buffer in chemistry?
A buffer is a solution that can resist pH change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus maintaining the pH of the solution relatively stable. This is important for processes and/or reactions which require specific and stable pH ranges.
What is the purpose of the buffer quizlet?
The function of a buffer is to resist changes in the pH of a solution when acid (HCl) or base (NaOH) (small amount) is added.
What is protein buffer?
Protein Buffers
Protein buffer systems depend upon proteins, as opposed to nonprotein molecules, to act as buffers and consume small amounts of acid or base. The protein hemoglobin makes an excellent buffer. It can bind to small amounts of acid in the blood, helping to remove that acid before it changes the blood’s pH.
What is a physiological buffer?
Physiological Buffers are chemicals used by the body to prevent sudden, rapid changes in the pH of a fluid. As explained in our discussion of the Henderson-Hasselbalch Equation, buffers are most able to resist changes in pH when the pH of the solution is close the unique pK of the buffer.
Why is a buffer important?
A buffer is a chemical substance that helps maintain a relatively constant pH in a solution, even in the face of addition of acids or bases. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis.
What is buffer and pH?
PH buffers are special solutions which prevent large variations in pH levels. Every pH level produced has a specified buffer capacity and buffer range. The capacity of the buffer refers to the amount of acid or base which can be added before the pH alters substantially.
What is pH full form?
The full form of pH is Potential of Hydrogen. pH is known as the negative logarithm of H+ ion concentration. Hence the meaning of the name pH is explained as the strength of hydrogen. pH describes the concentration of the hydrogen ions in a solution and it is the indicator of acidity or basicity of the solution.
What is a pH buffer biology?
Biological Buffers and pH Level
Biological buffers are organic substances that maintain a constant pH over a given range by neutralizing the effects of hydrogen ions.
Can amino acids function as buffers?
An amino acid can act as a buffer because it can react with added acids as well as to keep the pH nearly constant. Because an amino acid has both an acidic group which is a carboxyl group and a basic group which is an amine group, hence it can act as both acid and as a base therefore amino acids can act as a buffer.
What is the pH range of most buffer systems?
Buffers are generally good over the range pH = pKa ± 1. The ammonia buffer would be effective between pH = 8.24 – 10.24. The acetate buffer would be effective of the pH range from about 3.74 to 5.74. Outside of these ranges, the solution can no longer resist changes in pH by added strong acids or bases.
Do buffers release hydrogen ions?
Chemical buffers in the body are substances that can absorb extra hydrogen ions preventing a change in pH. For example, during exercise muscle cells can produce excess lactic acid, which increases hydrogen ions (acids release hydrogen ions).
How do buffers work in the human body?
A variety of buffering systems permits blood and other bodily fluids to maintain a narrow pH range, even in the face of perturbations. A buffer is a chemical system that prevents a radical change in fluid pH by dampening the change in hydrogen ion concentrations in the case of excess acid or base.
What are the main buffers in the body?
The body’s chemical buffer system consists of three individual buffers: the carbonate/carbonic acid buffer, the phosphate buffer and the buffering of plasma proteins. While the third buffer is the most plentiful, the first is usually considered the most important since it is coupled to the respiratory system.
In what way is a buffer important to living things?
In living organisms, buffers are important because they resist sudden changes in the pH of body fluids of living organisms: Bicarbonate buffer maintains the pH of the blood. Phosphate buffer maintains the internal environment of cells. Hemoglobin has buffering capacity.
What is a buffer made from?
Buffers can be made from weak acids or base and their salts. For example, if 12.21 grams of solid sodium benzoate are dissolved in 1.00 L 0.100 M benzoic acid (C6H5COOH, pKa = 4.19) solution, a buffer with a pH of 4.19 will result: Buffers can be made from two salts that provide a conjugate acid-base pair.
Do buffers increase or decrease pH?
The buffer range is the pH range where a buffer effectively neutralizes added acids and bases, while maintaining a relatively constant pH. The equation for pH also shows why pH does not change by much in buffers.
Are amino acids buffers?
An amino acid can act as a buffer because it can react with added acids as well as to keep the pH nearly constant. Because an amino acid has both an acidic group which is a carboxyl group and a basic group which is an amine group, hence it can act as both acid and as a base therefore amino acids can act as a buffer.
Is hemoglobin a buffer?
The most significant buffer of blood is hemoglobin. Thus, Harper (1967), Guyton (1968), Slonim A. Hamilton (1976) and other authors believe that it accounts for 50-60 percent of the total buffer capacity of blood.
Why are proteins buffers?
Proteins are made up of amino acids, which contain positively charged amino groups and negatively charged carboxyl groups. The charged regions of these molecules can bind hydrogen and hydroxyl ions, and thus function as buffers.